- Home
- Inclusions info
Inclusions types
Inclusions in Gemology
Inclusions studies
- Inclusions photo galleries
- Inclusions shop
- Shopping info
- Additional info
- Contact us
Raman spectroscopy
Introduction
Raman spectroscopy is a high resolution spectroscopic technique which allows us to identify almost any material in a matter of seconds. The analysis is based on the examination of the light scattered by the sample when it is traversed by a monochromatic light beam. A very small portion of this light changes in color (therefore in wavelength), this is the so called Raman effect. This wavelength shifts are characteristic for each material, so they allow us to recognize it.
With the development of the spectrometers and the computer-based interpretation of the results, as well as many variations to the original technique, according to the purpose and the conditions of each analysis, Raman spectroscopy has become one of the most important analytical methods in many different fields, such as chemistry, biology, medicine, archeology, materials science and, of course, gemology.
How it works
As it was said before, Raman spectroscopy is based on the Raman effect or Raman scattering, named after the Indian physicist Chandrasekhara Venkata Rāman, who first theorized it in 1928 (and received the Nobel Prize for Physics in 1930 for his discovery). Raman observed that, when light traverses a transparent material, nearly all of the light is dispersed without changing its wavelength (Rayleigh dispersion), but a very small portion of the light (one of every 10^7 photons) is scattered with a different wavelength. This is because the incoming light interacts with the vibrating molecules of the sample, causing them to jump to a virtual higher energetic level, and then back to a lower level, emitting a photon. If the photon is emitted at the same frequency as the incoming light (aka molecule returns to its original state), Rayleigh scattering is produced, if its frequency is lower (aka higher wavelength), Raman Stokes scattering is caused. If its frequency is higher, it is called Raman anti-Stokes scattering.
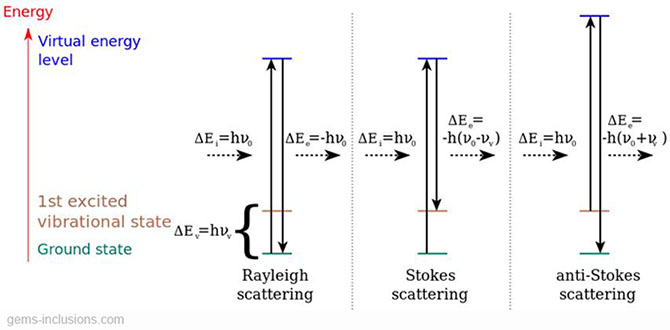
Each material produces a certain set of symmetrical Stokes and anti-Stokes shifts in the traversing light’s wavelength, determined by its atomic structure and composition, and independent of the incoming wavelength. Raman spectroscopy basically consists of studying the wavelength changes produced by the sample (its Raman fingerprint) and comparing them to established libraries of known materials. Stokes scattering is always much stronger than anti-Stokes, that is why the spectrometer is usually set to receive only higher wavelengths than the original laser beam.
Two main pieces of equipment are needed to perform a Raman spectroscopy: a laser emitter and a spectrometer. The first one sends a laser beam to the sample, and the second one collects the scattered light measures it’s the intensity for each wavelength. The two most common laser wavelengths used for Raman spectroscopy are 785 nm infrared and 532 nm green lasers. In theory, the wavelength of laser should not change amount of shift from original wavelength. 532 nm lasers produce a stronger Raman scattering, but also induce luminescent reactions, which can be diagnostic for the material but can hide Raman scattering too, as they are usually stronger. 785 nm lasers produce purer Raman spectra.
Spectral lines produced by Raman scattering are too weak to be seen by naked eye; a spectrometer or spectroscope is required. A spectrometer is a device used to measure the properties (usually intensity) of the incident light over a specific portion of the electromagnetic spectrum. Modern spectrometers consist of an optical system formed by a diffraction grafting and some mirrors and lenses, and a photodetector. The task of the optical system is to collect the scattered light and guide it to the photodetector, which converts the incident light into a digital signal, in order to send it to a computer. CCDs (Charge-coupled devices) are most used nowadays as photodetectors. Spectrometers covering a wide range of electromagnetic frequencies have been developed since the first visible light spectroscope, built in 1819, in order to work in many different fields like astronomy. The ones used for Raman spectroscopy only need to be sensitive to near-infrared, visible and near UV frequencies.
Until the 1990 decade, scanning spectrometers have dominated Raman spectroscopy. They were quite big and heavy equipment, which collected the light with a photographic film and presented the results in a spreadsheet. In the 90s, miniature spectrometers were developed. As their name suggests, they are smaller than scanning spectrometers (barely de size of a shoe box), and cheaper too. They use CCD sensors (or optical fiber in newer ones) to collect the light and send the information directly to a computer, which is displayed on the screen, and special software can automatically compare the results with a database of materials in order to identify the sample. Miniature spectrometers are often manufactured together with the laser emitter in a single device, which makes them safer and easier to use. Although, bigger spectrometers with more complex optical systems are still in use for carrying out very accurate analysis.
Gemmoraman-532 portable Raman-PL spectrometer, by M&A Gemological Instruments Ltd.
The results are usually presented in a graph like the one in the picture below. The x-axis contains the information about the received light wavelength. It represents the Raman shift between the incident monochromatic light beam and the scattered light. The light that traverses the sample without experimenting Raman scattering would be represented at 0 on this axis. It is always showed in terms of wavenumbers. Wavenumber is a magnitude which represents the inverse of the wavelength in cm, so its unit is cm-1. It is used rather than nanometers mainly because in wavenumbers, the Raman peaks are always the same, no regardless of the wavelength of the incoming laser. The Y-axis shows the intensity of the collected light for each wavelength. There is no information right after 0 in the X-axis because it is hard to filter the light that has not suffered Raman scattering, which is much more intense than the Raman peaks. That is why Raman spectrum typically starts from somewhere between 60 and 350 cm-1, depending on the laser filtering capacity of the spectrometer.
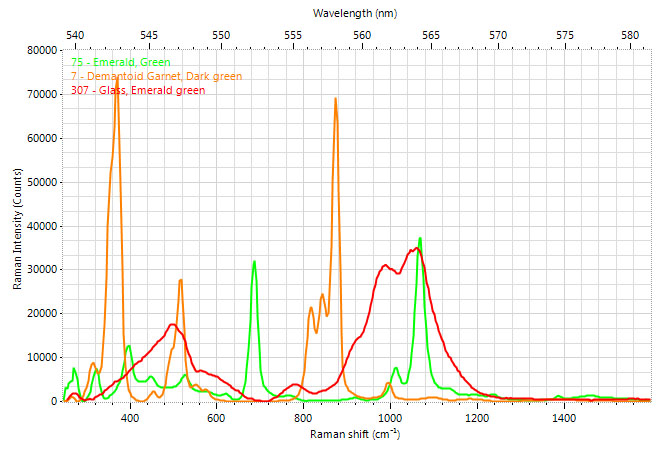
Example of Raman spectra of three different green materials – emerald, demantoid garnet and artificial glass, from the reference library of Gemmoraman-532 portable Raman-PL spectrometer.
Pros and cons
Raman spectroscopy is one of the most appropriate analyzing techniques for gemology. The main reason for this is its simplicity and quickness. With the modern miniature spectroscopes and the dedicated software, a jeweler can identify a stone (or an imitation) within a minute or two, without having a real gemological knowledge. This is because no sample preparation is needed for Raman spectroscopy (samples can even be analyzed through glass or polymer packaging, which is useful in other fields, like testing the composition of a medicine without unpacking it). It is also relevant that most of the Raman spectrometers can also perform luminescence analysis.
Of course, an experienced analyzer can extract much more complete information from Raman spectroscopy. For example, changes in Raman peak’s width can tell us about the amount of deformation of the material, which is very useful in engineering. Some other facts which can be acquired from Raman spectrum are the symmetry and orientation of a crystal, or its stress/strain state. This makes Raman spectroscopy a very complete analysis, especially if a high grade Raman spectroscope is used.
The main reason why Raman spectroscopy is so suitable for inclusions study is that there is no need for the studied object to be on the surface of the stone. This fact, along with its very high resolution (with the right optics, only a particle of less than 1 micrometer of diameter is needed), and its capability for being used for solids, liquid and even gases, makes Raman spectroscopy ideal for this purpose.
As any other technique, Raman spectroscopy has its limitations. In gemological analysis, the most important are that it doesn’t tell whether the material is natural or synthetic, or if any special treatments have been done (aside from some exceptions). It is also remarkable that, even though Raman spectroscopy is a non destructive technique, the laser beam can damage some materials, especially as its wavelength gets shorter.
References and links
- Gardiner, D.J. (1989). Practical Raman spectroscopy.
- Ferraro J. R., Nakamoto K. (2003). Introductory Raman Spectroscopy.
- Lewis, I.R.; Edwards, H.G.M. (2001). Handbook of Raman Spectroscopy.
- McCreery, R.L. (2000). Raman Spectroscopy for Chemical Analysis.
- Journal of Raman Spectroscopy
- http://en.wikipedia.org/wiki/Raman_spectroscopy
- http://gemmoraman.com/Articles/Ramandemystified.aspx
- http://www.ccmr.cornell.edu/igert/modular/docs/Appl_of_Raman_Spectroscopy.pdf